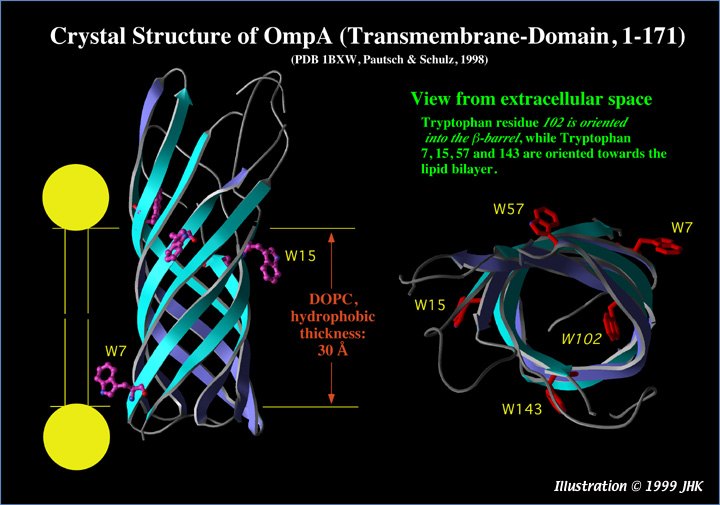

Structure of the transmembrane part of OmpA (residues 1-171, from Pautsch, A., Schulz, G. E., 1998, Nature Struct. Biol. 5, 1013-1017, PDB entry 1BXW).
A. Side view: This truncated OmpA (1-171) forms an 8-stranded β-barrel as previously predicted for the transmembrane domain of the complete OmpA (1-325) protein (Vogel and Jähnig, 1986, J. Mol. Biol. 190, 191-199). The 5 Trp residues of the protein are highlighted. The orientation shown does not necessarily reflect the real orientation of OmpA (1-325) in the membrane, because the angle between the barrel and the membrane normal is not precisely known. While the structure of wt-OmpA (1-325) is not yet published, it is very likely that residues 172-325 form a periplasmic domain. Fluorescence quenching experiments on single Trp mutants of OmpA (1-325), in which four out of five Trps were replaced by Phenylalanine, showed Tryptophan residues 15, 57, 102, and 143 at 10 Å on the trans-side and Trp-7 at 10 Å on the cis side of a DOPC bilayer (Kleinschmidt et al., 1999, Biochemistry, 38 5006-5016).
An animated version of the side view of OmpA on the left hand side is available here (2 MB Quicktime file, Quicktime 3.0, http://quicktime.apple.com/sw/sw3.html , is required).
B. Top view. Trp-102 is oriented towards the inside of the β-barrel. The other Tryptophan residues are oriented towards the membrane lipids. For an animated version of the top view, click here (2.7 MB Quicktime 3.0 file)
A few more crystal strucures of other β-barrel outer membrane proteins can be found here.